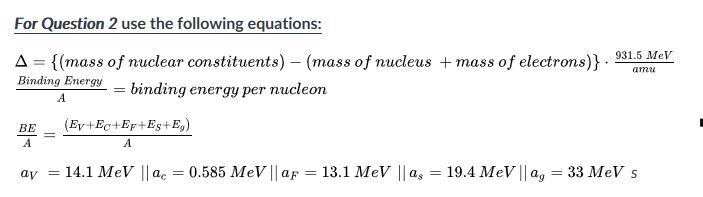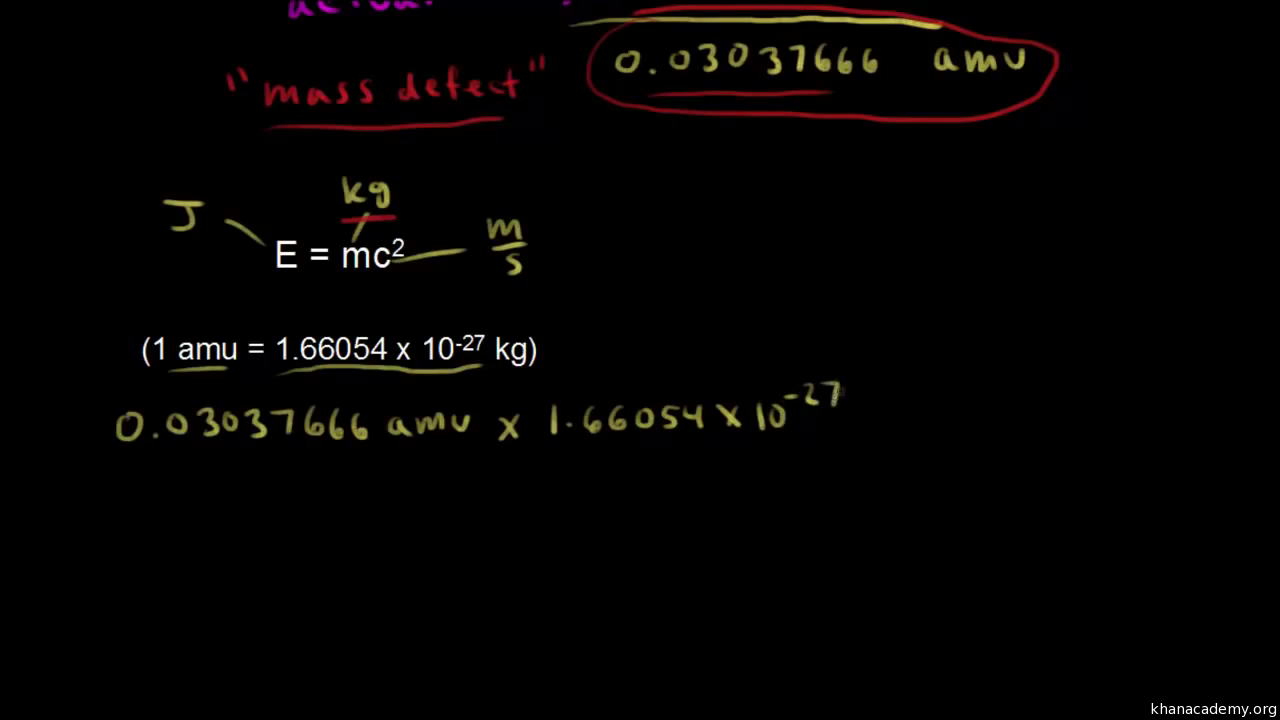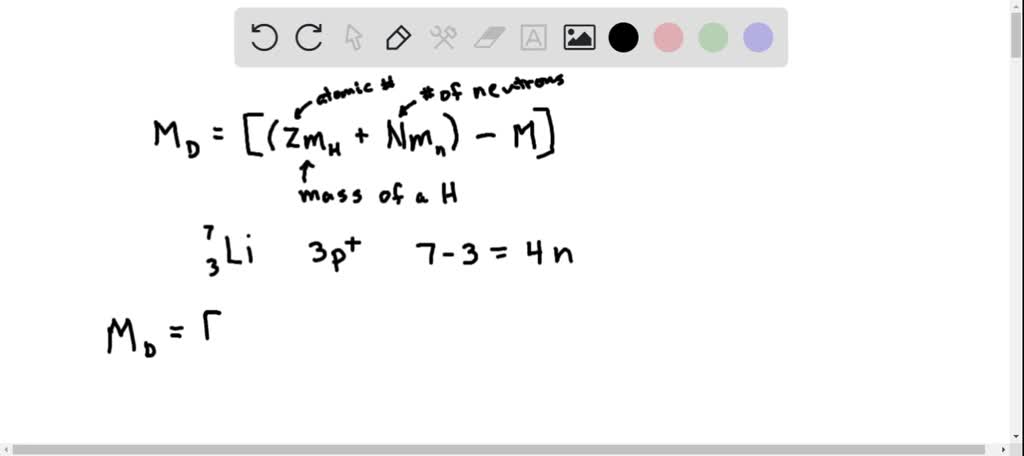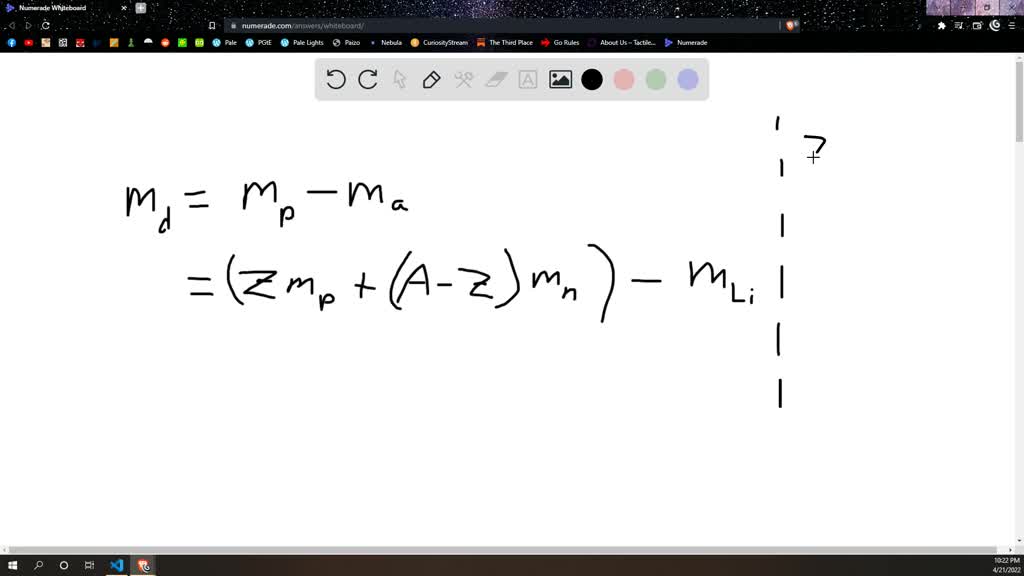
SOLVED: Calculate the mass defect for lithium-7. The mass of lithium-7 is 7.016003 amu. (1 proton = 1.007826 amu, 1 neutron = 1.008665 amu) A 7.016 amu B 7.058 amu C 0.0421 amu D 4.035 amu
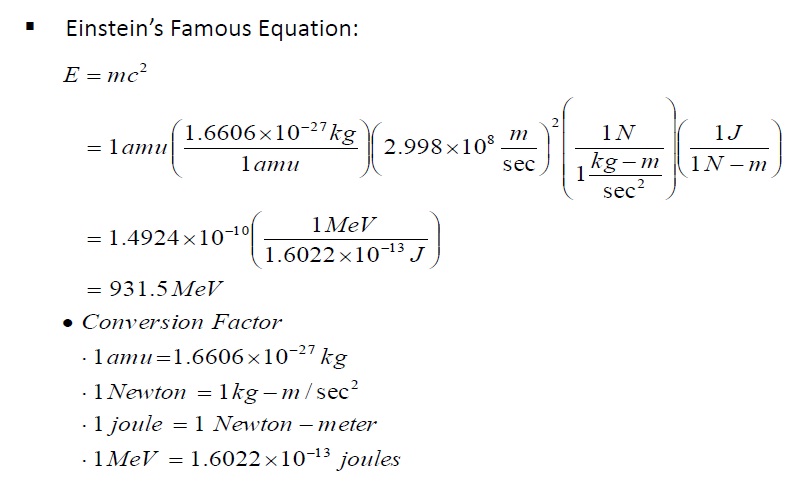
In nuclear physics, what is meant by the term 'mass defect', and what is its relation to nuclear energy? | Socratic
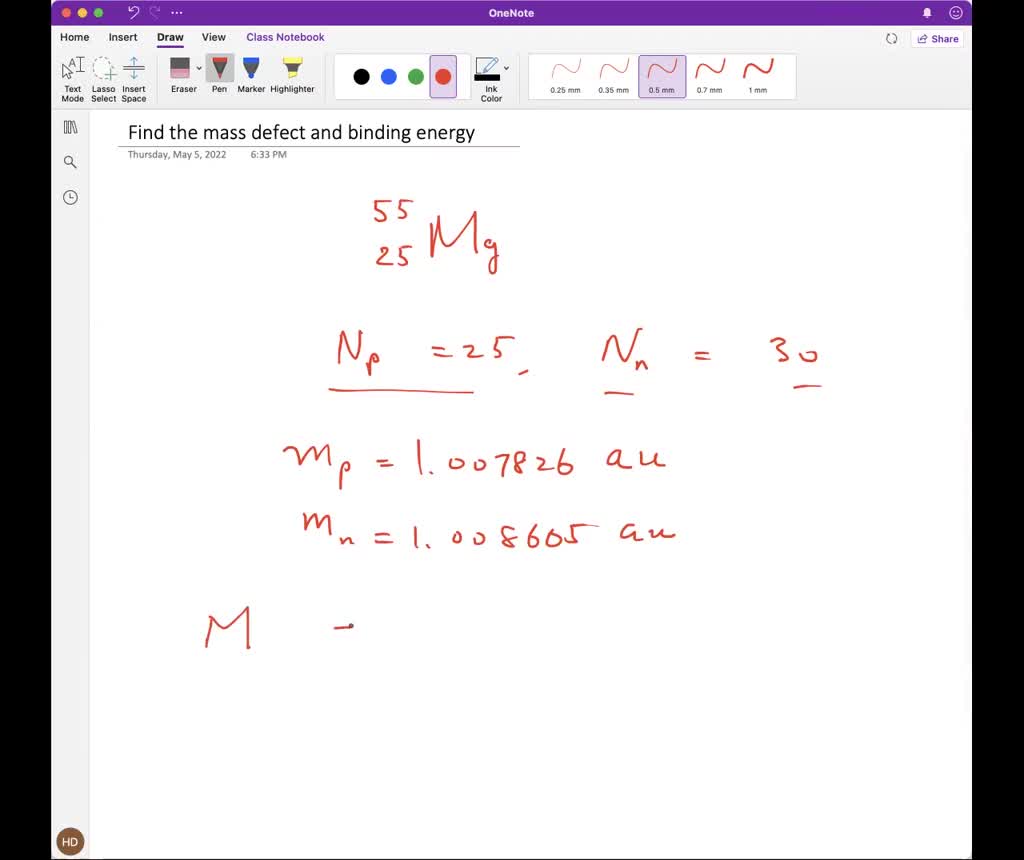
SOLVED: Find the mass defect and binding energy for Manganese with A= 55 Z-25 the mass of it is 54.938044 amu mass of proton = 1.007826 amu, mass of neutron = 1.008665 amu mass of electron = 0.00054859 *
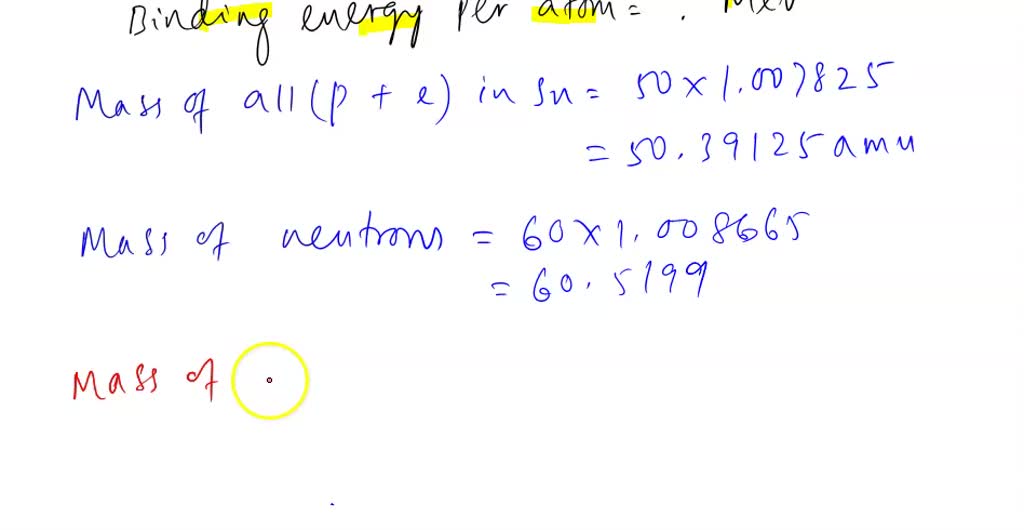
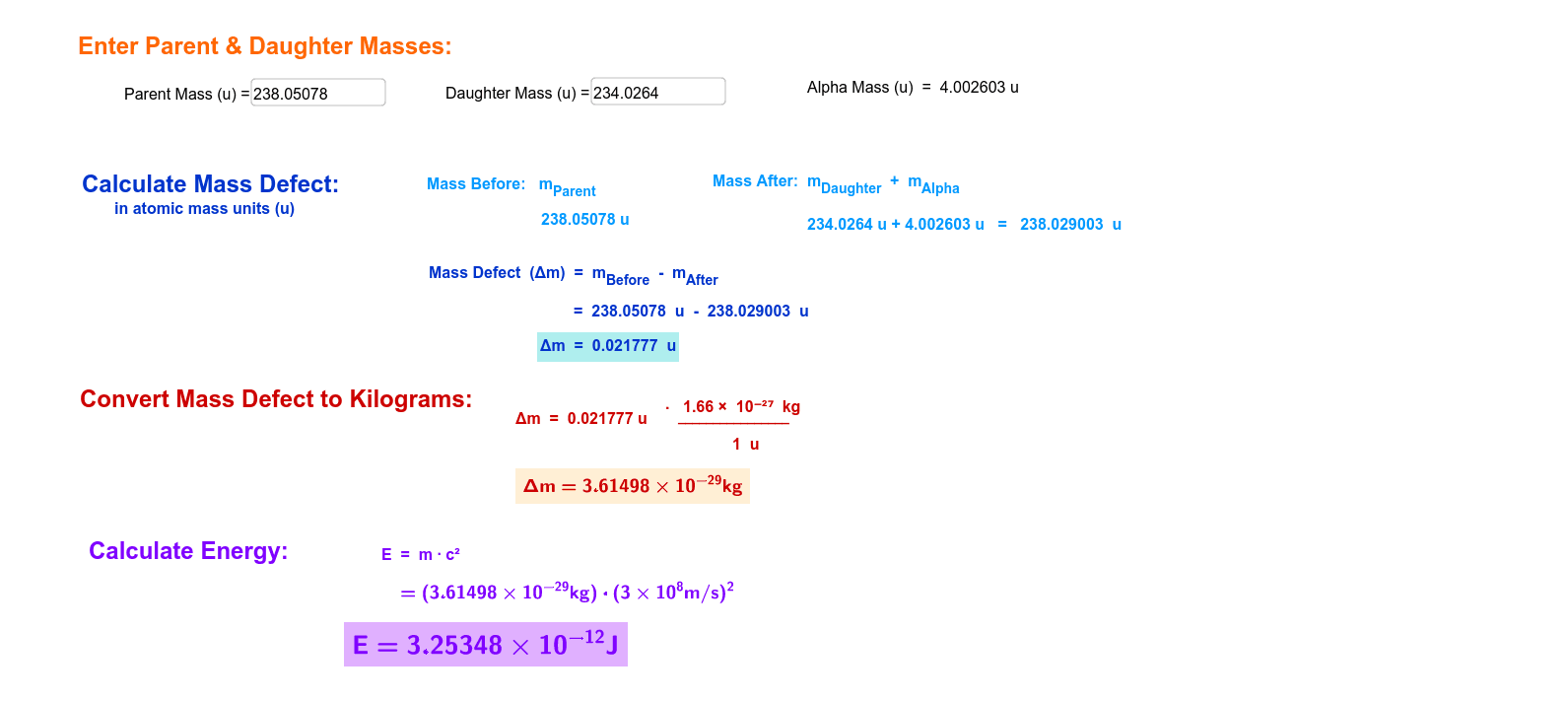
SOLVED: QUESTION 4 An atom of 110Sn has mass of 109.907858 amu: Calculate the binding energy in MeV per atom: Use the masses: mass of 'H atom 1.007825 amu mass of a

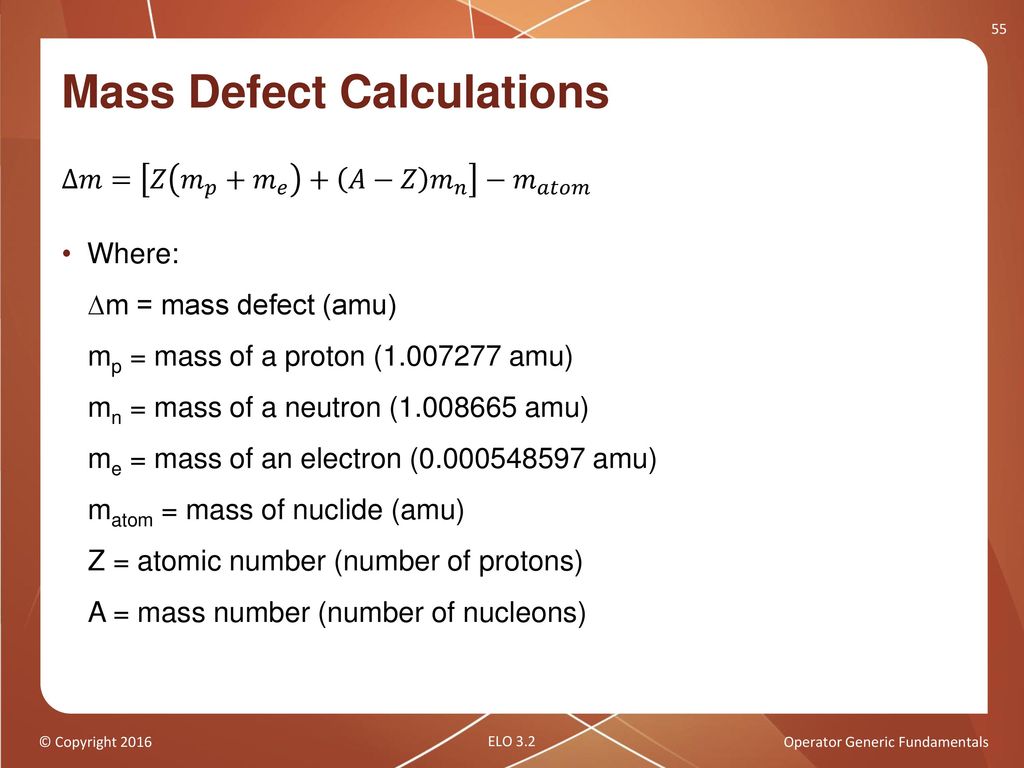
Mass Defect Formula & Examples | What is Nuclear Mass Defect? - Video & Lesson Transcript | Study.com

Calculate mass defect, binding energy and binding energy per nucleon for a lithium nucleus (.3Li^7) taking its mass =7.000000a.m.u. Mass proton=1.007825 a.m.u. and mass of neutron =1.008665 a.m.u. Take 1a.m.u. =931.5 MeV.

OpenStax College Physics Solution, Chapter 28, Problem 48 (Problems & Exercises) | OpenStax College Physics Answers

Calculate the (i) mass defect, (ii) binding energy and (iii) the binding energy per nucleon for a 6C^12 nucleus. Nuclear mass of 6C^12 = 12.000000 a.m.u., mass of hydrogen nucleus = 1.007825